
The most advanced non-invasive prenatal test (NIPT) that uses groundbreaking technologies.
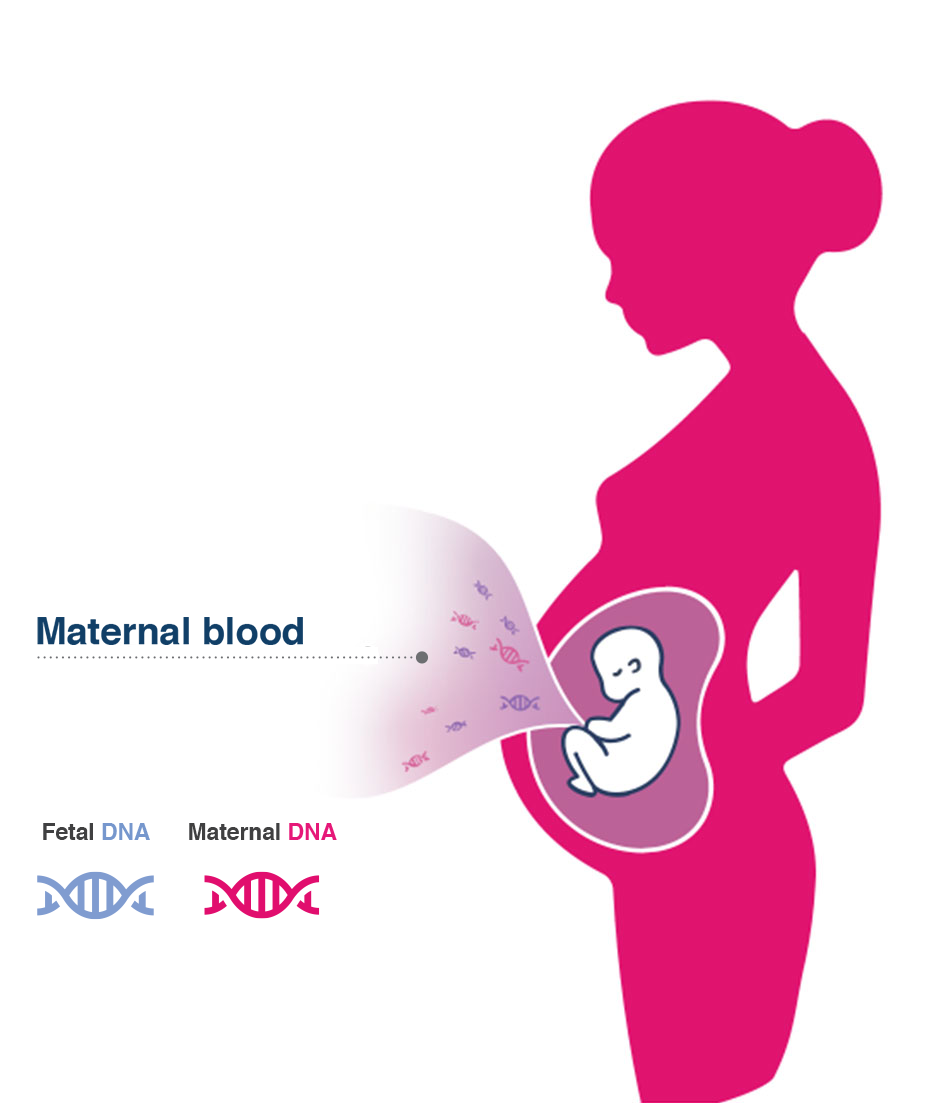
Through analysis of circulating cell-free fetal DNA (cfDNA) in maternal blood, it screens multiple genes to assess severe inherited and de novo genetic disorders in the fetus, that are not screened for with current NIPT technology.

During pregnancy placental trophoblasts, through a physiological process known as "apoptosis", releases DNA fragments in the maternal blood. This DNA, also named circulating cell-free fetal DNA, increases with advancing of gestational age and, at the 10th week of gestation, reaches a sufficient quantity for a reliably analysis and provides valuable information on the health of the fetus.
INDICATION FOR TESTING


is particularly indicated for patients who meet any of the following criteria:

Pregnancy at risk for a genetic condition screened

Abnormal ultrasound finding(s) suggestive of a genetic disorder screened

Patients wishing to avoid an invasive diagnostic procedure

Advanced paternal age (men that are >40 years old)
The test is suitable for every kind of pregnancy: singleton or twin pregnancy, natural conception or IVF derived pregnancy, pregnancy at risk for an inherited genetic disorder or to any pregnancy without a specific a priori risk
SCREENING LEVELS
A CUTTING-EDGE NIPT THAT OFFERS A FULL PANEL OF SCREENING OPTIONS


geneadvance
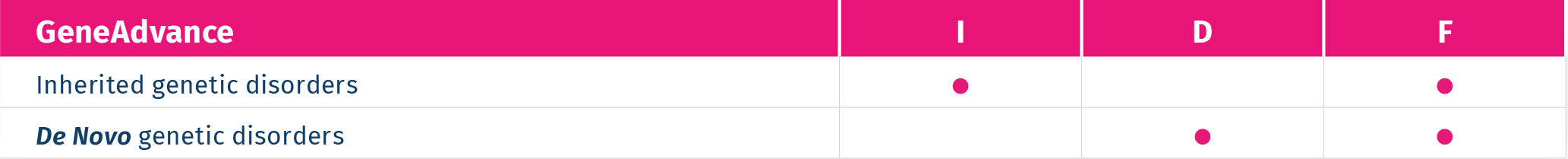
inherited
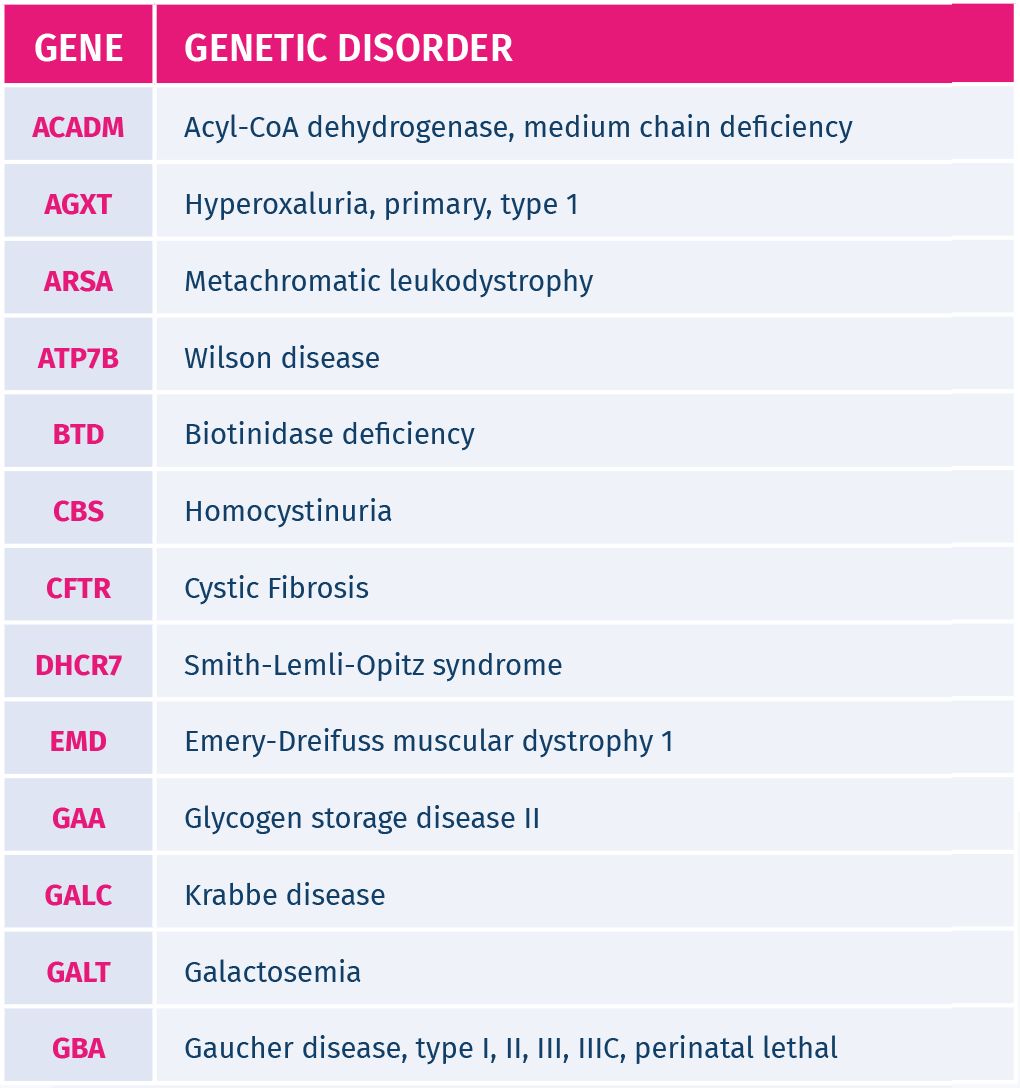
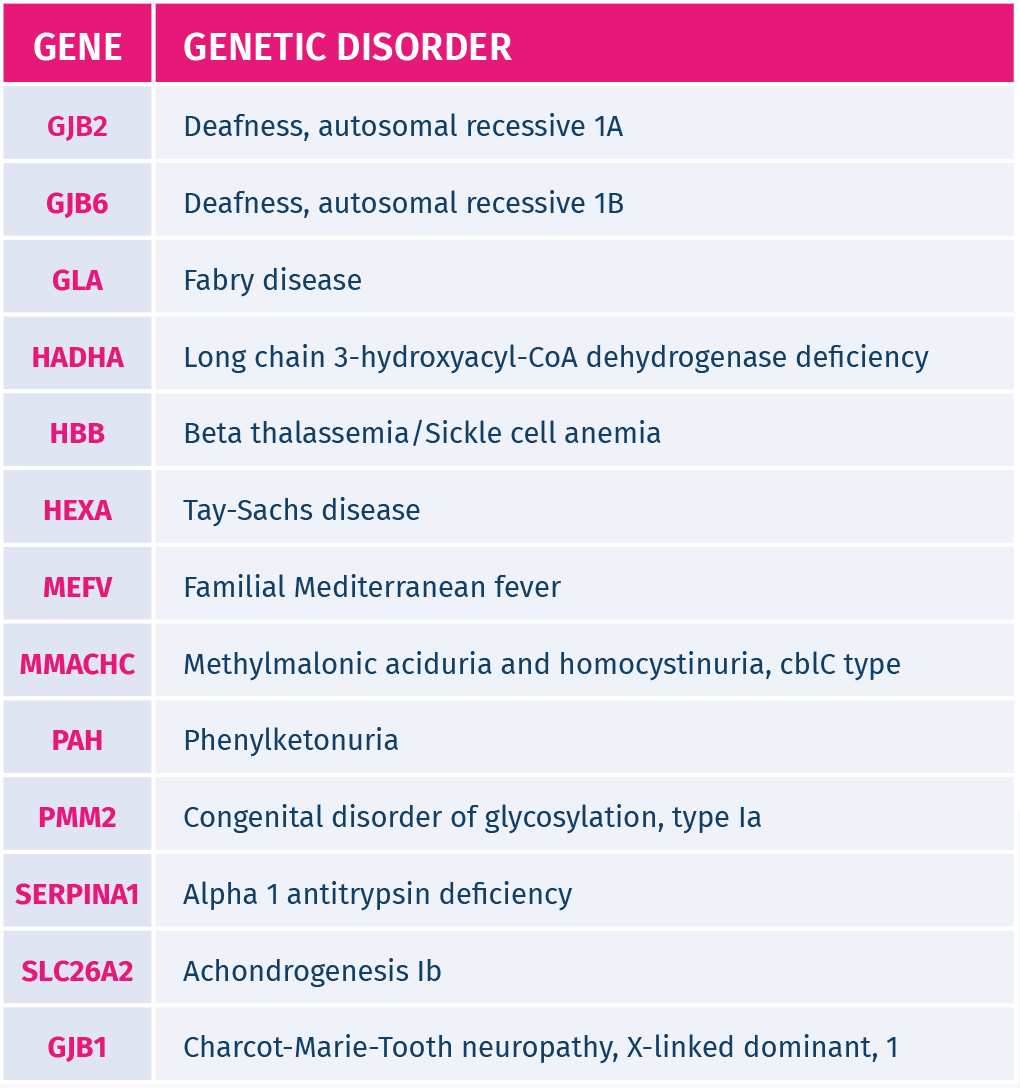
It screens for 26 common inherited recessive genetic disorders, such as Cystic Fibrosis, Beta Thalassemia, Sickle cell anemia, Autosomal recessive deafness 1A, Autosomal recessive deafness 1B.

geneadvance
de novo
It screens for 53 severe genetic disorders due to de novo mutations (a gene mutation that is not inherited) in 35 genes that cause skeletal dysplasia, congenital heart defects, multiple congenital malformation syndromes, neurodevelopmental disorders and sporadic cases of various rare dominant Mendelian disorders, such as Schinzel- Giedion syndrome and Bohring-Opitz syndrome.

geneadvance
fullscreen
It facilitates early diagnosis of severe genetic conditions, allowing detection in the fetus of 26 inherited disorders and 53 with de-novo on-set, that could be missed by traditional prenatal screening.
COMPARING THE LEVELS

COMPARING THE LEVELS



IT ALLOWS IDENTIFICATION OF SEVERE FETAL INHERITED GENETIC CONDITIONS

The inherited genetic disorders screened by GeneAdvance Inherited test are the most common in the European population

IT ALLOWS IDENTIFICATION IN THE FETUS OF GENE MUTATIONS CAUSING SEVERE DE NOVO GENETIC CONDITIONS

De novo genetic conditions screened in the fetus:
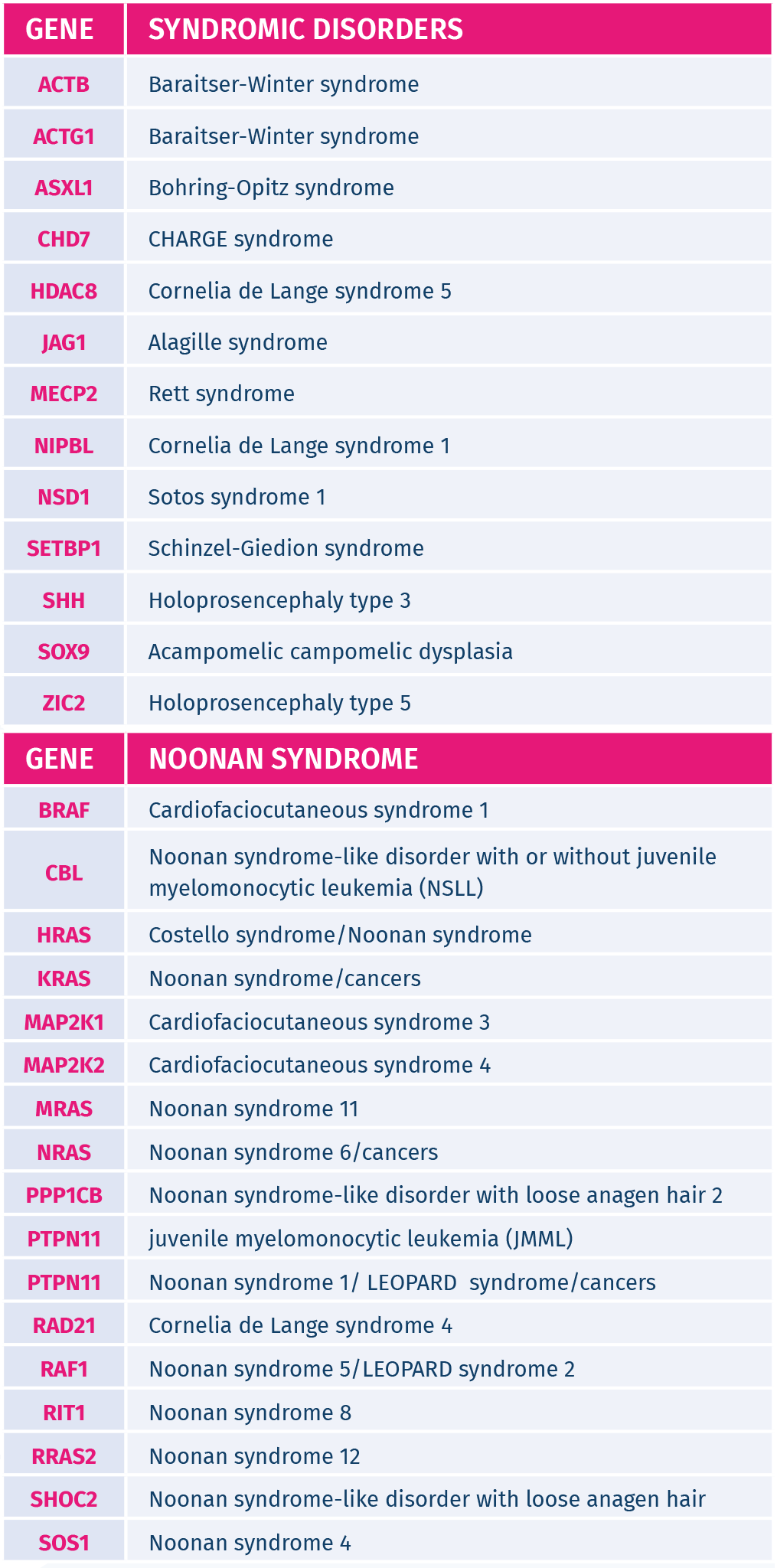
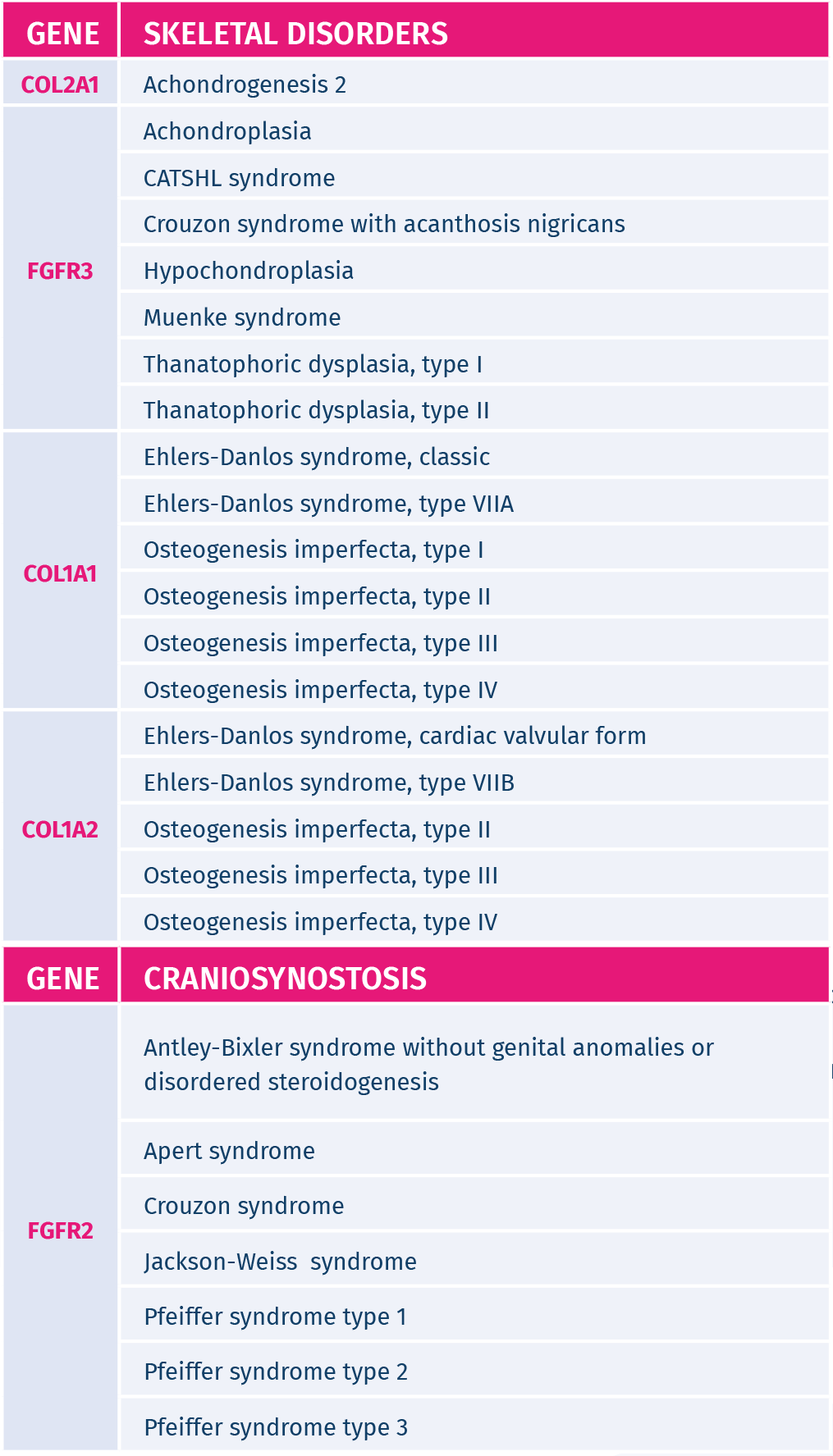
GeneAdvance De Novo test detects de novo mutations in 35 genes causing 53 different genetic disorders. The genetic conditions screened by this innovative test often occur in the absence of a family history of the condition and cannot be detected by standard carrier screening, as these mutations are not present on the parents. The genetic disorders screened by GeneAdvance De Novo can cause skeletal dysplasias, cardiac defects, multiple congenital anomalies, autism, epilepsy and/or intellectual disability.
IDENTIFIES FETAL GENETIC CONDITIONS THAT ARE NOT TYPICALLY ASSOCIATED WITH ABNORMAL ULTRASOUND FINDINGS

Many of the genetic disorders screened by the GeneAdvance De Novo test are not detectable by prenatal ultrasound investigations (especially in the first trimester). Some of these genetic diseases are only detectable in the late second or third trimester or after delivery. These diseases can lead to serious conditions such as skeletal dysplasias, heart defects, multiple congenital anomalies and / or intellectual disability in the child.

SCREENS FOR GENETIC DISEASES ASSOCIATED WITH ADVANCED PATERNAL AGE

Traditional non-invasive prenatal screening tests detect fetal chromosomal abnormalities typically associated with advanced maternal age (eg Down syndrome). The GeneAdvance De Novo test also identifies genetic diseases associated with advanced paternal age (eg. Achondroplasia, Pfeiffer's syndrome, Apert's syndrome, Crouzon's syndrome, Osteogenesis lmperfecta, etc.), caused by DNA mutations that arise during the spermatogenesis process, providing couples with advanced age the opportunity to use a more comprehensive screening test. GeneAdvance De Novo test allows identification of fetal genetic conditions whose incidence is independent of maternal age. Genetic diseases detectable with this test have a cumulative incidence ~1/600, and ~1/300 for disorders determining developmental delay..
2. McRae J, et aL Prevalence and architecture of de novo mutatians in develapmental disarders. Nature 542. "33--438
HOW IT WORKS
A GROUNDBREAKING TECHNOLOGY ALLOWING

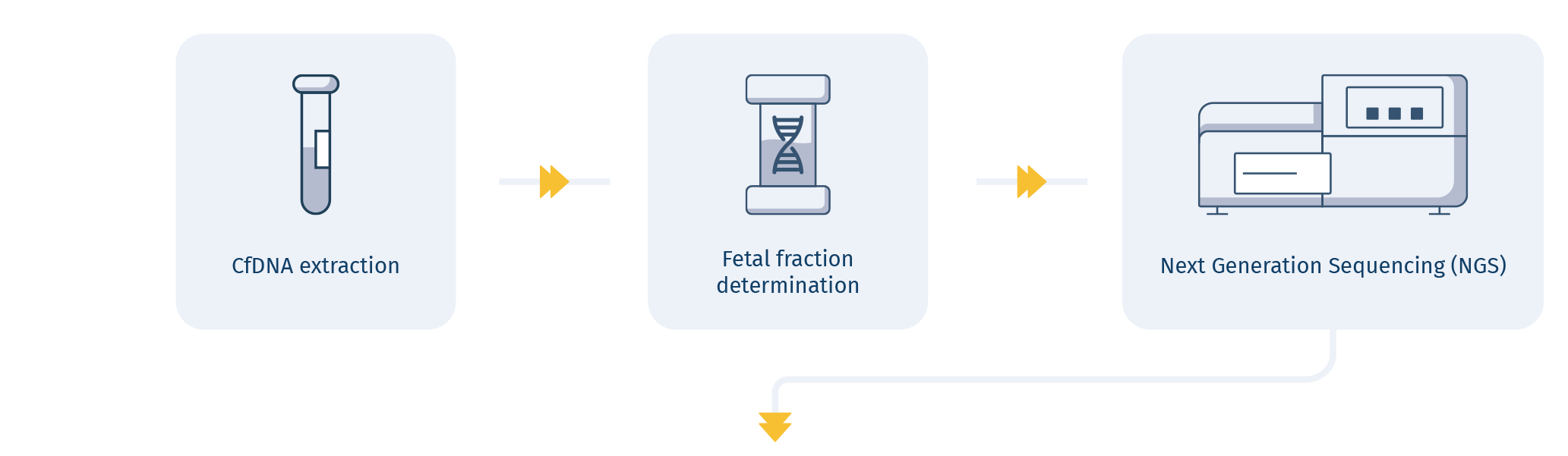
CfDNA mutation analysis

A groundbreaking technology coupled with an advanced bioinformatic analysis allows for detection of DNA mutation causing serious genetic disorders, providing the most comprehensive information available from a noninvasive prenatal test to date

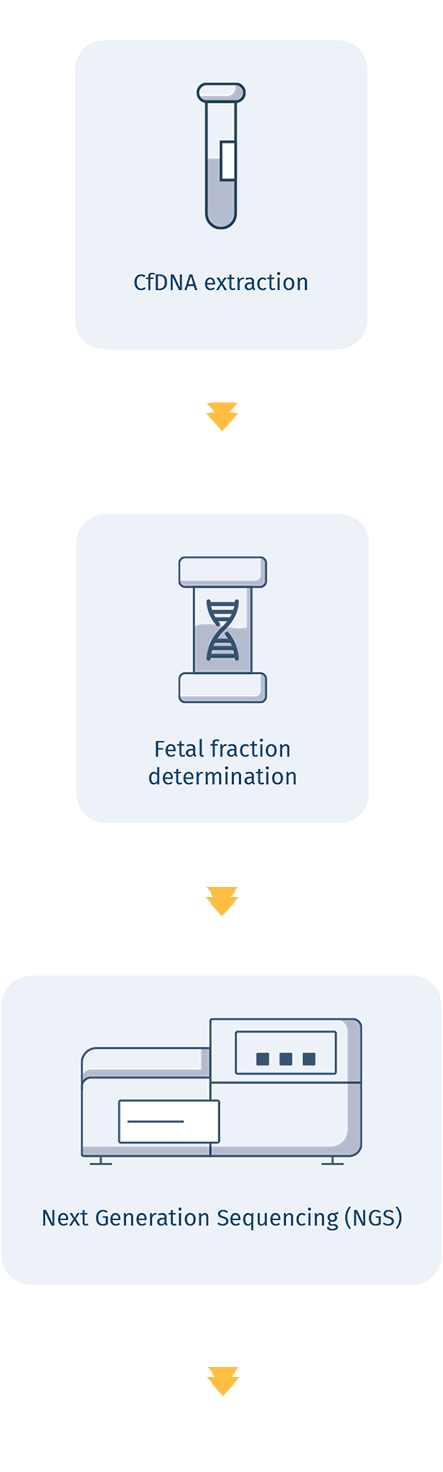
CfDNA mutation analysis

A groundbreaking technology coupled with an advanced bioinformatic analysis allows for detection of DNA mutation causing serious genetic disorders, providing the most comprehensive information available from a noninvasive prenatal test to date..

TEST RESULTS



NEGATIVE
Non-pathogenic/likely pathogenic mutation(s) detected: this result indicates that no disease causing mutations have been detected in the targeted genes screened. In this case, the pregnancy is considered to be at low risk for the genetic diseases investigated. A low risk test result greatly reduces the chances that the fetus is affected by a monogenic disorder but it cannot guarantee a healthy baby.

POSITIVE
Pathogenic/Likely Pathogenic mutation(s) detected: this result indicates that one or more disease causing mutations have been detected in the targeted genes screened. In such cases, pregnancy is considered to be at high risk and requires follow-up by invasive prenatal diagnosis (amniocentesis or CVS) for confirmation of test results, before any medical decisions are made. A patient with a positive test result should be referred for genetic counseling.

NEGATIVE
Non-pathogenic/likely pathogenic mutation(s) detected: this result indicates that no disease causing mutations have been detected in the targeted genes screened. In this case, the pregnancy is considered to be at low risk for the genetic diseases investigated. A low risk test result greatly reduces the chances that the fetus is affected by a monogenic disorder but it cannot guarantee a healthy baby.

POSITIVE
Pathogenic/Likely Pathogenic mutation(s) detected: this result indicates that one or more disease causing mutations have been detected in the targeted genes screened. In such cases, pregnancy is considered to be at high risk and requires follow-up by invasive prenatal diagnosis (amniocentesis or CVS) for confirmation of test results, before any medical decisions are made. A patient with a positive test result should be referred for genetic counseling.

SERVICES INCLUDED
- Free follow-up of abnormal results
- Reimbursement of the test fee for cases with fully inconclusive test results
- Pre- and Post-test genetic counselings
SUPERIOR ACCURACY AND HIGH QUALITY STANDARDS


Simple
A simple blood sample (8-10 ml) collected at 10^ weeks of gestation is required

Fast
Turnaround time of ~10 days

Safe
It is a non-invasive prenatal test, no risk for the fetus and the mother

Sensitive
Low limit of detection: highly accurate at low cfDNA quantity (FF:2%)

Reliable
Sensitivity and specificity >99%

Comprehensive
Performed in combination with PrenatalAdvance test, detects both genome-wide chromosomal abnormalities and single-gene disorders, providing the most comprehensive information available from a noninvasive prenatal test to date
4 Easy Steps

WHY CHOOSE


ADVANCED MOLECULAR DIAGNOSTICS SOLUTIONS USING STATE-OF-THE ART TECHNOLOGIES
GENOMICA is recognized as one of the most advanced molecular diagnostics laboratory in Europe, both for the state-of-the-art instruments and technologies, as well as for its high quality standards. With a comprehensive portfolio of over 10.000 genetic tests, GENOMICA is able to satisfy increasingly specialised requests in the field of molecular genetics, providing physicians and their patients with innovative and highly specialised diagnostic solutions for any clinical need

Over 100.000
genetic tests/year

Test performed in Italy
(Rome or Milan)

20+ years experience in prenatal molecular diagnostics

Fast
TAT

Dedicated
R&D team

Laboratories with groundbreaking technologies and high quality standards

Personalized genetic counseling with genetic counselors experts in discussing genetic test results and familial risks

International
Partnership
WHY CHOOSE


ADVANCED MOLECULAR DIAGNOSTICS SOLUTIONS USING STATE-OF-THE ART TECHNOLOGIES
GENOMICA is recognized as one of the most advanced molecular diagnostics laboratory in Europe, both for the state-of-the-art instruments and technologies, as well as for its high quality standards. With a comprehensive portfolio of over 10.000 genetic tests, GENOMICA is able to satisfy increasingly specialised requests in the field of molecular genetics, providing physicians and their patients with innovative and highly specialised diagnostic solutions for any clinical need

Over 100.000 genetic tests/year

Test performed in Italy
(Rome or Milan)

20+ years experience
in prenatal molecular diagnostics

Fast
TAT

Dedicated
R&D team

Laboratories with groundbreaking technologies and high quality standards

Personalized genetic counseling with genetic counselors experts in discussing genetic test results and familial risks

International
Partnership
Information request for GENEADVANCE test
Fill out this form for a free consultation.
One of our professionals will contact you, free of charge and without obligation, to provide you with all the information you need.



 Italiano
Italiano English
English